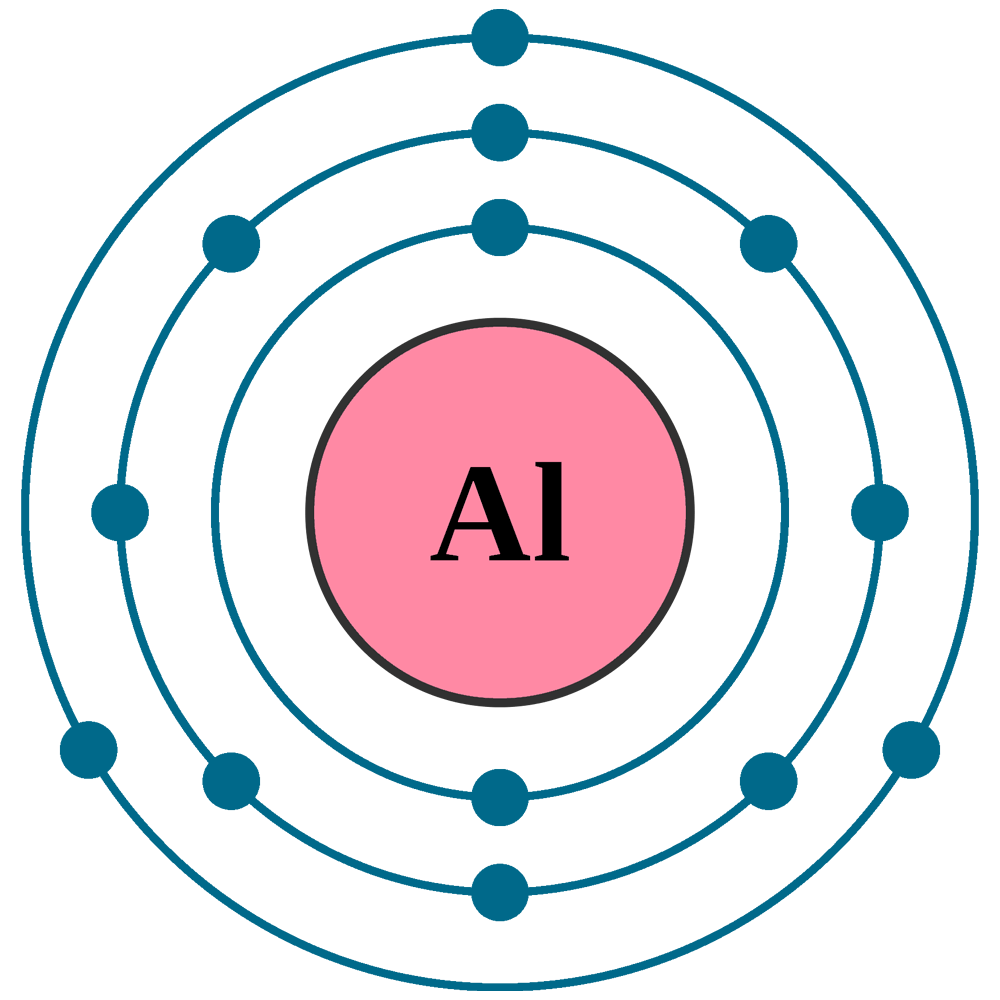
Draw The Electron Configuration For A Neutral Atom Of Aluminum. - The next six electrons can go in the 2p orbital.
Draw The Electron Configuration For A Neutral Atom Of Aluminum. - We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): Thus, it is simple to determine the charge on such a negative ion: The atomic number of al is 13. Web electron configuration of oxygen (o) [he] 2s 2 2p 4: Electron configuration of neon (ne) [he] 2s 2 2p 6:
\[\dot{al:} \nonumber \nonumber \] the valence electron configuration for selenium is 4s 2 4p 4. Web the electron configuration for aluminum is: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Energy this problem has been solved! An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation. The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l), and
Atomic structure of aluminum Brainly.in
Web an aluminum atom is a neutral atom that has an atomic number of 13 which implies it has a total of 13 electrons. Web the first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1 s2 2 s2 2 p6 3 s2.
SOLVED Draw the electron configuration for & neutral atom of aluminum
This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. 1s 2 2s 2 2p 6 3s 1: So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: Web write the full electron configuration.
Bohr Model Electron Aluminium Lewis Structure Atom, PNG, 600x590px
Web electron configuration of aluminium is [ne] 3s2 3p1. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. This video discusses how to write electron configurations for h, li, c and sc. This problem has been solved! Web the easiest way to create.
Aluminium Al (Element 13) of Periodic Table Elements FlashCards
Web electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of sulfur. The first two electrons can go in the 1s orbital. Web faq this electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element.
Diagram representation of the element aluminium Vector Image
The atomic number of al is 13. The next six electrons can go in the 2p orbital. An atom has a valence shell electron configuration of #ns^1#. 1s 2 2s 2 2p 6: This tells you that the electron configuration of a neutral aluminium atom must account for a total of #13# electrons. This problem.
Atom Diagrams Electron Configurations of the Elements
Web in order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). We describe an electron configuration with a symbol that contains three pieces of information (figure 6.25): You'll get a detailed solution from a subject matter expert that helps you.
How Can We Find Electron Configuration For AL (Aluminium)
1s 2 2s 2 2p 5: Web the block that the atom is in (in the case for aluminum: Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Web configuration of an atom draw the electron configuration for a neutral atom of aluminum. Web faq.
Aluminium electronic configuration How to Write Aluminium electronic
Energy this problem has been solved! \[\dot{al:} \nonumber \nonumber \] the valence electron configuration for selenium is 4s 2 4p 4. 1s 3p 2p 2s 3s this problem has been solved! The electron configuration of aluminum is 1s^2 2s^2 2p^6 3s^2 3p^1 to draw the electron configuration put al in the middle of a circle.
Electron arrangements
Electron configuration of neon (ne) [he] 2s 2 2p 6: Electron configuration of sodium (na) [ne] 3s 1: Aluminum is the 13th element on the periodic table, so it has 13 electrons in a neutral state. Web an electrically neutral atom has the following electron configuration: Web an electron configuration diagram is a model that.
How Can We Find Electron Configuration For Aluminium (Al)
Web an electrically neutral atom has the following electron configuration: Web the block that the atom is in (in the case for aluminum: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The atomic number of al is 13. Web step 1/6 1. \[\dot{al:} \nonumber \nonumber \] the.
Draw The Electron Configuration For A Neutral Atom Of Aluminum. So now we have 2s². Web the electron configuration for aluminum is: Web electron configuration of aluminium is [ne] 3s2 3p1. The next six electrons can go in the 2p orbital. However, a curious thing happens after the 3 p subshell is filled:
We Describe An Electron Configuration With A Symbol That Contains Three Pieces Of Information (Figure 6.25):
The charge is equal to the number of electrons that must be gained to fill the s and p. As per the aufbau rule, the electrons will be filled into 1s orbital first then 2s, then 2p…so on. We'll represent this as 1s². Aluminium is located in period 3, group 13, and has an atomic number equal to #13#.
Web Faq This Electron Configuration Calculator Will Instantly Show You The Distribution Of Electrons In The Orbitals Of Any Periodic Element You Choose.
So now we have 2s². Web the easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. When we write the configuration we'll put all 13 electrons in orbitals around the nucleus of the aluminium atom.
Web Draw The Electron Configuration For A Neutral Atom Of Aluminum.
Web in order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13 electrons). 1s^2 2s^2 2p^6 3s^2 3p^1 to figure out the electron configuration of any element you will use the diagonal diagram (seen in the right side of video below) and use the aufbau principle. Web electronic structure drawing a box diagram of the electron configuration of an atom draw the electron configuration for a neutral atom of sulfur. Web your starting point here will be the electron configuration of a neutral aluminium atom, #al#.
However, A Curious Thing Happens After The 3 P Subshell Is Filled:
The number of the principal quantum shell, n, the letter that designates the orbital type (the subshell, l), and Web write the full electron configuration for a neutral aluminum atom. An atom has a valence shell electron configuration of #ns^1#. This problem has been solved!





:max_bytes(150000):strip_icc()/aluminiumatom-58b602655f9b5860464c6f7d.jpg)



