Draw The Lewis Structure For The Bromine Difluoride Ion - Web brf2 lewis structure is drawn depending on the valence electrons of br and f atoms.
Draw The Lewis Structure For The Bromine Difluoride Ion - 1)know the total number of valence electrons present total number of valence electrons present in brf 2 is 21 (7 electrons from bromine and 14 electrons from 2 fluorine atoms). Web draw lewis structures for covalent compounds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Bromine is the least electronegative and goes at the center of the brf 2 lewis structure.; Web several lewis structures can be written for perbromate ion, , the central br with all single br—o bonds, or with one, two, or three br=o double bonds.
Determine the total number of valence electrons in the molecule or ion. Determine the total number of valence electrons in the molecule/ion. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. There are a few steps that need to be followed to attain the stable and correct lewis structure which are as follows: Web several lewis structures can be written for perbromate ion, , the central br with all single br—o bonds, or with one, two, or three br=o double bonds. So, if you are ready to go with these 5 simple steps, then let’s dive right into it! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Bromine Facts, Symbol, Discovery, Properties, Uses
Web how to draw lewis structure for bromine. Determine the total number of electrons in the valence shells of bromine atoms. Determine the total number of valence electrons in the molecule or ion. Fluorine is a group 17 element on the periodic table. This means that the central bromine (br) atom will have an odd.
Solved Draw the Lewis structure of the bromine difluoride
Each h atom (group 1) has 1 valence electron, and the o atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. There are a few steps that need to be followed to attain the stable and correct lewis structure which are as follows: There are 4 single bonds between the.
Lewis structure Bromine pentafluoride Sulfur tetrafluoride Xenon
1)know the total number of valence electrons present total number of valence electrons present in brf 2 is 21 (7 electrons from bromine and 14 electrons from 2 fluorine atoms). The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. Valence electron, hybridization, solubility,.
BrF2 Lewis Structure How to Draw the Lewis Structure for Bromine
Draw the lewis structure of the bromine difluoride cation, brf2+ do not include brackets or formal charges. Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). With brf 2 there are an odd number of valence electrons (21 total). Determine the.
How to Draw the Lewis Dot Structure for BrF2 YouTube
Draw the lewis structures of these possible resonance structures, and use formal charges to predict which makes the greatest contribution to the resonance hybrid. Bromine can hold more than 8 valence electrons since it is. The bromine molecule contains only one element. You'll get a detailed solution from a subject matter expert that helps you.
Bromine Formula \({\rm{B}}{{\rm{r}}_2}\) Structure, Molar Mass, IUPAC
The following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. With brf 2 there are an odd number of valence electrons (21 total). Web draw lewis structures for covalent compounds. For the brf2 structure use the periodic table to find the total number of.
BrF3 Lewis Structure (Bromine Trifluoride) YouTube
Brf2 is also termed as bromo difluoride. Bromine is the least electronegative and goes at the center of the brf 2 lewis structure.; The following procedure can be used to draw lewis structure for simple molecules. Web draw the lewis dot structure of the triiodide ion , and explain what conditions /reactants are necessary for.
Bromine Lewis Dot Structure Drawing, Several Compounds and Detailed
Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). 1)know the total number of valence electrons present total number of valence electrons present in brf 2 is 21 (7 electrons from bromine and 14 electrons from 2 fluorine atoms). Web draw.
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy
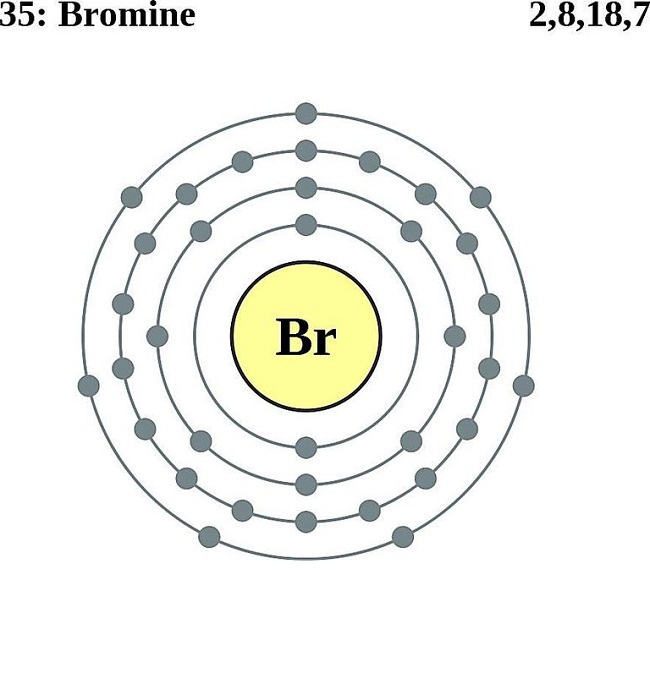
Web drawing lewis structures for molecules with one central atom: Web [1] hence, the valence electrons present in bromine is 7 (see below image). If the molecule exhibits resonance, draw only one (1) of the resonance forms. There are a few steps that need to be followed to attain the stable and correct lewis structure.
Lewis Dot Diagram For Bromine Wiring Diagram
Web brf2 lewis structure is drawn depending on the valence electrons of br and f atoms. Web draw lewis structures for covalent compounds. With brf 2 there are an odd number of valence electrons (21 total). Determine the total number of valence electrons in the molecule/ion. The bromine molecule contains only one element. Determine the.
Draw The Lewis Structure For The Bromine Difluoride Ion For the brf2 structure use the periodic table to find the total number of valence electrons. Web several lewis structures can be written for perbromate ion, , the central br with all single br—o bonds, or with one, two, or three br=o double bonds. Determine the total number of valence electrons in the molecule/ion. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. There are 4 single bonds between the bromine atom (br) and each fluorine atom (f).
Lewis Structures Are Representations Of Molecules That Include Not Only What Atoms Are Present In The Molecule But Also How The Atoms Are Connected.
Valence electron, hybridization, solubility, covalent nature and polarity of brf2 is also explained. Web draw the lewis structure for the bromine difluoride (brf2) ion. There are 4 single bonds between the bromine atom (br) and each fluorine atom (f). Web draw the lewis dot structure of the triiodide ion , and explain what conditions /reactants are necessary for it to form this problem has been solved!
The Following Procedure Can Be Used To Construct Lewis Electron Structures For More Complex Molecules And Ions.
Web draw lewis structures for covalent compounds. Web brf2 lewis structure is drawn depending on the valence electrons of br and f atoms. Bromine is the least electronegative and goes at the center of the brf 2 lewis structure.; Brf2 is also termed as bromo difluoride.
[2] Hence, The Valence Electrons Present In Fluorine Is 7 (See Below Image).
You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Lewis diagram of xenon difluoride (xef₂) in some molecules, the central atom exceeds the octet rule (is surrounded by more than eight electrons). There are a few steps that need to be followed to attain the stable and correct lewis structure which are as follows: This means that the central bromine (br) atom will have an odd number (9 total).
See An Example Of A Molecule That Violates The Octet Rule (Xef₂) And Learn How To Draw Its Lewis Diagram In This Video.
Determine the total number of valence electrons in the molecule or ion. 1)know the total number of valence electrons present total number of valence electrons present in brf 2 is 21 (7 electrons from bromine and 14 electrons from 2 fluorine atoms). Web drawing lewis structures for molecules with one central atom: Web several lewis structures can be written for perbromate ion, , the central br with all single br—o bonds, or with one, two, or three br=o double bonds.