How Do Atoms Form Chemical Bonds - The approximate shape of a molecule can be predicted from the number of electron groups and the number of surrounding atoms.
How Do Atoms Form Chemical Bonds - The approximate shape of a molecule can be predicted from the number of electron groups and the number of surrounding atoms. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of electrons as in covalent bonds. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. When atoms approach one another, their electrons interact and tend to distribute themselves in space so that the total energy is lower than it would be in any alternative arrangement. Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms.
The first way gives rise to what is called an ionic bond. Three idealized types of bonding are ionic bonding , in which positively and negatively charged ions are held together by electrostatic forces, covalent bonding , in which electron pairs are shared between atoms and metallic. Let’s begin… atoms can (and do) bond constantly; Figure 1a shows a protein, depicted in green, attached to a dna molecule, shown in orange. Web an ionic bond, where one atom essentially donates an electron to another, forms when one atom becomes stable by losing its outer electrons and the other atoms become stable (usually by filling its valence shell) by gaining the electrons. Chemical reactions occur when chemical bonds between atoms are formed or broken. Valence electrons are the electrons in the outer energy level of an atom that may be involved in chemical interactions.
Chemical Bonds · Anatomy and Physiology
Chemical bonds (exercises) these are exercises and select solutions to accompany chapter 9 of the beginning chemistry textmap formulated around the ball et al. It also is the smallest unit of matter that has the characteristic properties of a chemical element. It's how they form molecules. Web atoms form two basic bonds, covalent or ionic.
Metallic Chemical Bonds Educational Resources K12 Learning, Chemistry
Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Consider as an example an atom of sodium, which has one electron in its.
Ionic Bond Definition, Types, Properties & Examples
There are three different types of. We can therefore say that a molecule is the simplest unit of a covalent compound. Web chemical bonding is the general term used to describe the forces that hold atoms together in molecules and ions. Since the valence electrons are the outermost electrons, they have the greatest opportunity to.
chemical bonding Definition, Types, & Examples Britannica
Web atom, smallest unit into which matter can be divided without the release of electrically charged particles. Atoms form chemical bonds to achieve a full outer energy level, which is the most stable arrangement of electrons. Web chemical bonding is the general term used to describe the forces that hold atoms together in molecules and.
What Happens When Atoms Bond infographic diagram showing how electrons
Web a chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules, crystals, and other structures. Three idealized types of bonding are ionic bonding , in which positively and negatively charged ions are held together by electrostatic forces, covalent bonding , in which electron pairs are shared between atoms.
Chemical Bonds · Anatomy and Physiology
When a hydrogen atom loses its single electron. Let’s begin… atoms can (and do) bond constantly; Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species. The approximate shape of a molecule can be predicted from the number of electron groups and the number.
ionic bond Definition, Properties, Examples, & Facts Britannica
When atoms approach one another, their electrons interact and tend to distribute themselves in space so that the total energy is lower than it would be in any alternative arrangement. Web when atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. [a note on definitions] representing molecules: Since the.
PPT Why do atoms form bonds? PowerPoint Presentation, free download
Web the electrons constituting a chemical bond are simultaneously attracted by the electrostatic fields of the nuclei of the two bonded atoms. Web 32,968 questions answered. Web view full lesson: Atoms can also play nicely and share electrons in a covalent bond. There are three different types of. Web chemical bonding, any of the interactions.
Covalent bond Definition, Properties, Examples, & Facts Britannica
Web atom, smallest unit into which matter can be divided without the release of electrically charged particles. Web the atoms of molecules are linked together through a reaction known as chemical bonding. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more stable than when.
Chemistry B.Sc Level How many types of chemical bond
The columns of the periodic table, which contain elements that show a family resemblance, are called groups. Web atoms form two basic bonds, covalent or ionic bonds, to fill the full outer shell of electrons. Therefore, the valence electrons have the most influence in. Web atoms can join together by forming a chemical bond, which.
How Do Atoms Form Chemical Bonds The first way gives rise to what is called an ionic bond. In a homonuclear molecule such as o2 the bonding electrons will be shared equally by the two atoms. Web there are three basic ways that the outer electrons of atoms can form bonds: Life is based on chemical interactions let us start with an example of why understanding the properties of atoms and molecules is essential for understanding living systems. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more stable than when the atoms are apart.
Chemical Bonds (Exercises) These Are Exercises And Select Solutions To Accompany Chapter 9 Of The Beginning Chemistry Textmap Formulated Around The Ball Et Al.
Web why and how atoms bond together to form molecules. [a note on definitions] representing molecules: The columns of the periodic table, which contain elements that show a family resemblance, are called groups. Many atoms become stable when their valence shell is filled with electrons or when they satisfy the octet rule (by having eight valence electrons).
Web The Electrons Constituting A Chemical Bond Are Simultaneously Attracted By The Electrostatic Fields Of The Nuclei Of The Two Bonded Atoms.
Valence electrons are the basis of all chemical bonds. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full electron shell. Web chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other species. Web a chemical bond is a force of attraction between atoms or ions.
Therefore, The Valence Electrons Have The Most Influence In.
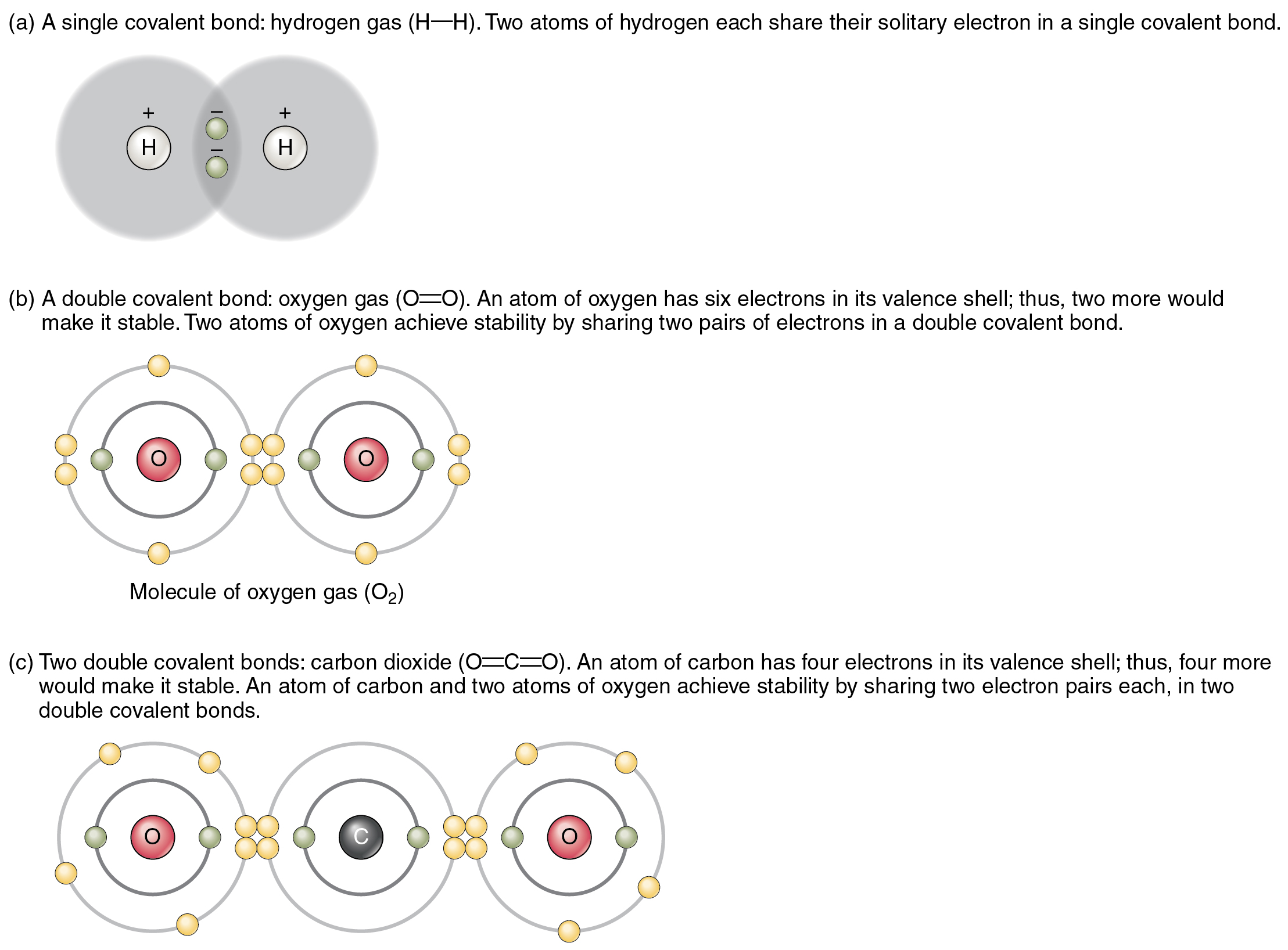
Web chemical bonding is the general term used to describe the forces that hold atoms together in molecules and ions. The potential energy of two separate hydrogen atoms (right) decreases as they approach each other, and the single electrons on each atom are shared to form a covalent bond. Life is based on chemical interactions let us start with an example of why understanding the properties of atoms and molecules is essential for understanding living systems. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more stable than when the atoms are apart.
Atoms Form Chemical Bonds To Achieve A Full Outer Energy Level, Which Is The Most Stable Arrangement Of Electrons.
It also is the smallest unit of matter that has the characteristic properties of a chemical element. Valence electrons are the electrons in the outer energy level of an atom that may be involved in chemical interactions. Web when atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. There are three different types of.