How Many Covalent Bonds Can Sulfur Form - Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule and only.
How Many Covalent Bonds Can Sulfur Form - Finally, if both of sulfur's. Group 6a form 2 bonds; A 70 kg (150 lb) human body contains about 140 grams of sulfur. The lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms.â this yields the expected. There is a quick way to work out how many covalent bonds an element.
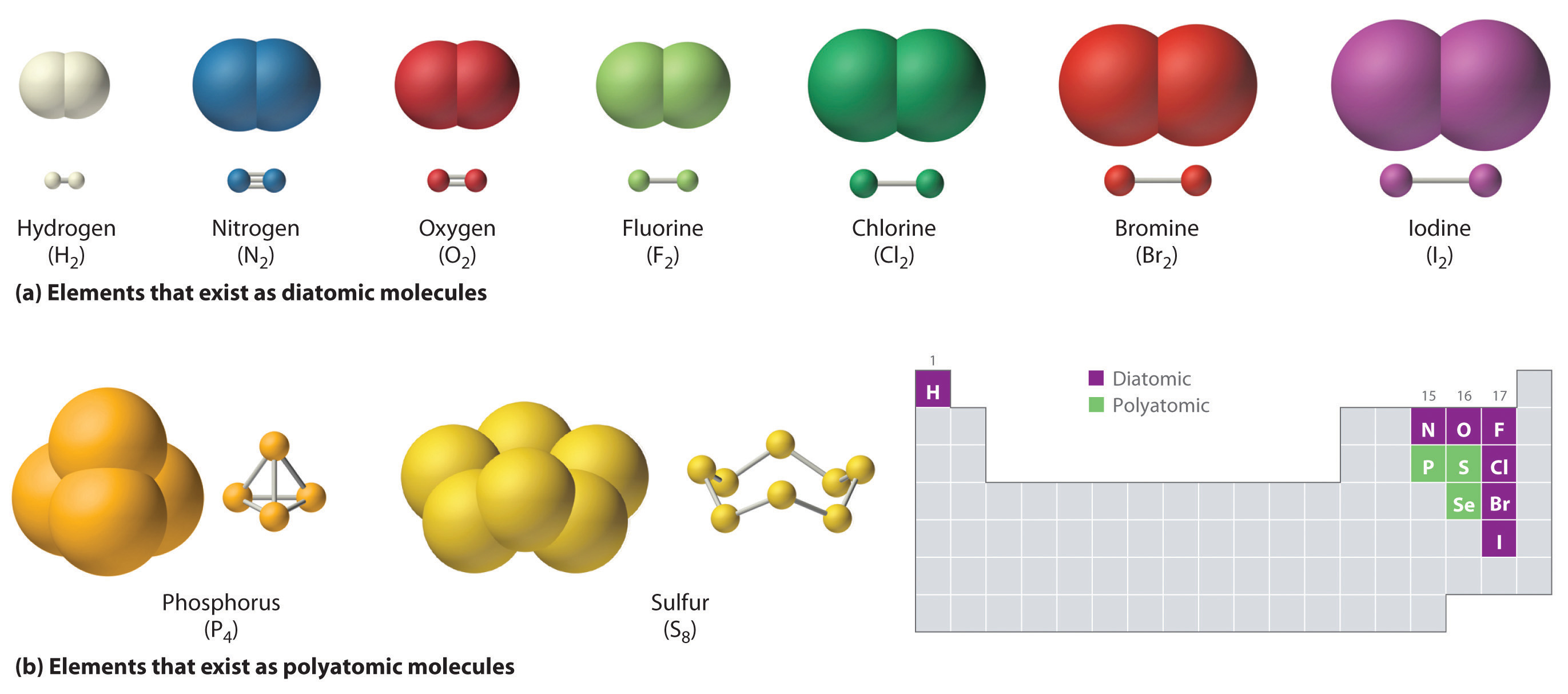
Web study with quizlet and memorize flashcards containing terms like if sulfur has an atomic number of 16, how many covalent bonds can it form with other atoms? Which of the following constitutes a covalent. Group 6a form 2 bonds; Web formation of covalent bonds. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web covalent radius half of the distance between two atoms within a single covalent bond. Group 5a form 3 bonds;
Given below shows the bonding structure of sulphuric acid.Explain even
Is determined by the distance at which the lowest potential energy is achieved. Is assigned as 2, since two hydrogens are required to satisfy bonding needs of these atoms. Web sulfur indeed can make up to 6 bonds. Web how many single covalent bonds can halogens form? Group 5a form 3 bonds; Web if one.
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of
Web now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. Is determined by the distance at which the lowest potential energy is achieved. Web the valence of oxygen, sulfur, etc. Is sulfur trioxide ionic or covalent? Because hydrogen only.
Molecules Free FullText Thiosulfoxide (Sulfane) Sulfur New
Group 6a form 2 bonds; Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Web covalent radius half of the distance between two atoms within a single covalent bond. Is assigned as 2, since two hydrogens.
Reading Covalent Bonds Biology I
It is the eighth most abundant element in the human body by weight, about equal in abundance to potassium, and slightly greater than sodium and chlorine. Values are given for typical oxidation number and coordination. How many covalent bonds can each carbon atom form? Because hydrogen only needs two electrons to fill its valence shell,.
Question 51110 Socratic
Sulfur is an essential component of all living cells. Web each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the phosphorus's unpaired electrons. Finally, if both of sulfur's. Group 5a form 3 bonds; Web oxygen and other atoms in group 6a (16).
covalent bond Definition, Properties, Examples, & Facts Britannica
Is determined by the distance at which the lowest potential energy is achieved. Nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web sulfur indeed can make up to 6 bonds. Web formation of covalent bonds. Web study with quizlet and memorize flashcards containing terms like if sulfur has an atomic number of 16,.
SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular Geometry
Group 6a form 2 bonds; Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Web up to 6% cash.
sulfur Definition, Element, Symbol, Uses, & Facts Britannica
Web up to 6% cash back how many single covalent bonds would the element sulfur be expected to form in order to obey the octet rule? Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web now sulfur has 6 unpaired electrons which means it can.
Why can sulfur bond 6 times?
Web covalent radius half of the distance between two atoms within a single covalent bond. The carbon atom is unique among elements in its tendency to form extensive networks of covalent. Group 6a form 2 bonds; Web how many single covalent bonds can halogens form? Web up to 6% cash back how many single covalent.
CH103 Chapter 5 Covalent Bonds and Introduction to Organic Molecules
Web if one of sulfur's electron pairs is separated, as illustrated in the middle structure in figure \(\pageindex{1}\), four covalent bonds can be created. Web each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the phosphorus's unpaired electrons. In fact, it is.
How Many Covalent Bonds Can Sulfur Form The potential energy of two separate hydrogen atoms (right) decreases as. Web how many single covalent bonds can halogens form? Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Values are given for typical oxidation number and coordination. Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule and only.
Finally, If Both Of Sulfur's.
Because hydrogen only needs two electrons to fill its valence shell, it is an exception to the octet rule and only. The lewis structure for the sulfate ion consists of a central sulfur atom with four single bonds to oxygen atoms.â this yields the expected. For example, the hydrogen molecule, h 2, contains a covalent bond. The carbon atom is unique among elements in its tendency to form extensive networks of covalent.
Group 5A Form 3 Bonds;
In fact, it is a multivalent atom, meaning it can make different number of bonds, up to 6, depending on the chemical formula. How many covalent bonds can each carbon atom form? Values are given for typical oxidation number and coordination. Web formation of covalent bonds.
It Is The Eighth Most Abundant Element In The Human Body By Weight, About Equal In Abundance To Potassium, And Slightly Greater Than Sodium And Chlorine.
Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around its valence shell. The octet rule states that atoms of all elements forms bond with other atoms in such a way that each atom has eight electrons (octet) in its outermost. Fluorine and the other halogens in group 7a (17) have seven valence electrons and can.
The Potential Energy Of Two Separate Hydrogen Atoms (Right) Decreases As.
Web up to 6% cash back how many single covalent bonds would the element sulfur be expected to form in order to obey the octet rule? Web how many bonds can sulfur form? Group 6a form 2 bonds; Web sulfur indeed can make up to 6 bonds.