Kr Electron Configuration Long Form - Web electron configuration of krypton is [ar] 3d10 4s2 4p6.
Kr Electron Configuration Long Form - We can now add the remaining electrons for kr. [kr]5s 2 4d 10 5p 5 Krypton has a total of 36 electrons second: The electron configuration of ar is: Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5. Large number of spectral lines, strongest being green and yellow/ whitish emission ; Web krypton(kr) electron configuration long form: Web by “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. The atomic mass of krypton is 83.798 u and its density is 3.75 g/l. This page shows the electron configurations of the neutral gaseous atoms in their ground states. Web the commonly used long form of the periodic table is designed to emphasize electron configurations.
Krypton Kr (Element 36) of Periodic Table Elements FlashCards
Los alamos national laboratory, u.s. [kr]5s 2 4d 10 5p 5 Large number of spectral lines, strongest being green and yellow/ whitish emission ; Web electron configuration of ruthenium (ru) [kr] 4d 7 5s 1. Kr is the symbol for krypton, which is a noble gas. 2, 8, 18, 16, 1. 1s 2 2s 2.
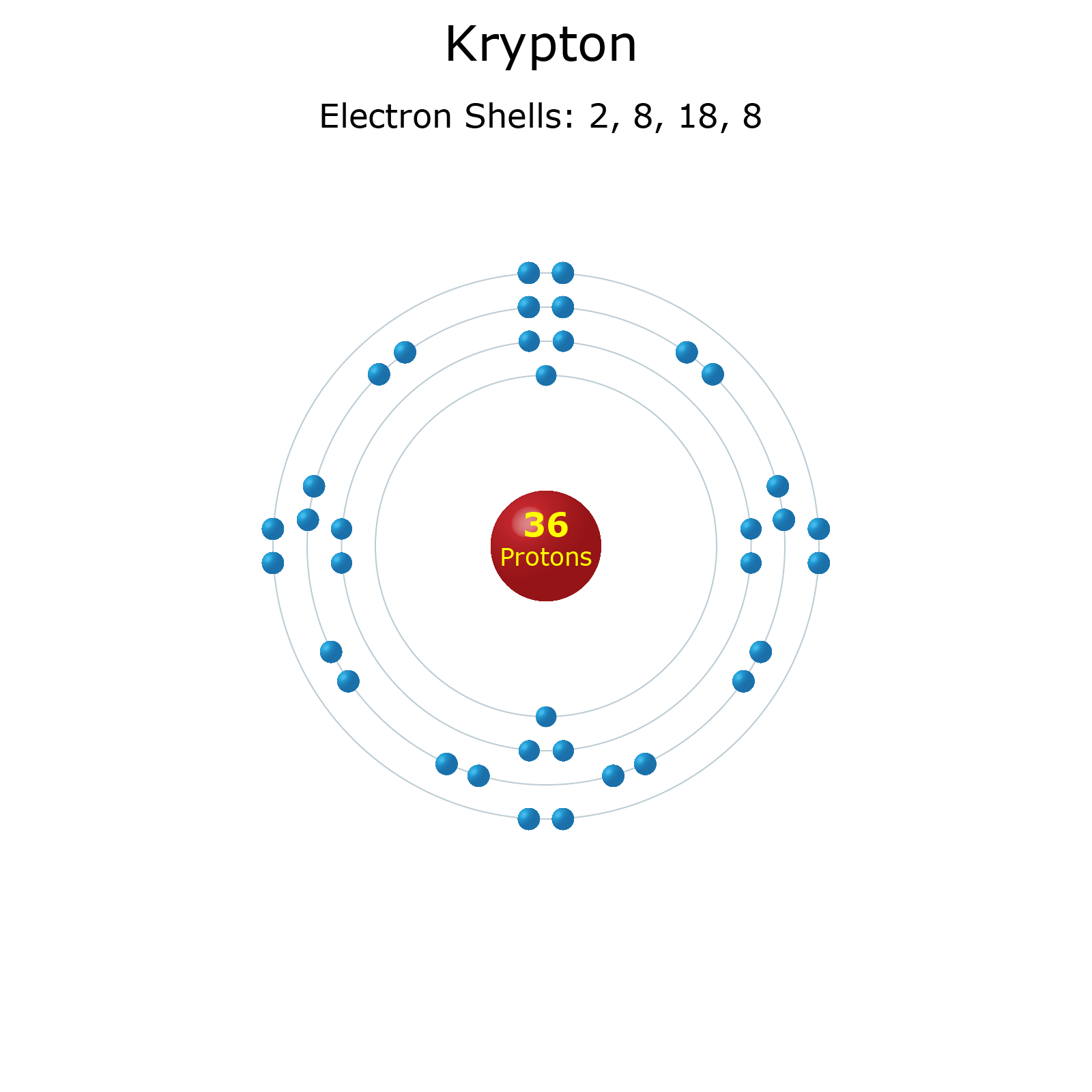
Krypton Periodic Table and Atomic Properties
Longhand electron configuration for krypton(kr): 1s^ (2)2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 or [kr] or [ar] 4s^2 3d^10 4p^6 first: Kr has 18 electrons in the 4th energy level (n=4), so we can add them to the electron configuration of ar: Web to help describe the appropriate notation for electron configuration, it is best.
Krypton Facts Atomic Number 36 Element Symbol Kr
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 8 5s 1. Web next, we need to determine the electron configuration of kr's noble gas predecessor, which is ar (atomic number 18). Kr is the symbol for krypton, which is a noble gas. Web physical properties of.
How to write the electron configuration for Krypton (Kr) YouTube
Web 2.2 electron configuration [ar]4s 2 3d 10 4p 6. We can now add the remaining electrons for kr. Therefore, the valence electrons of krypton are eight. The electron configuration of ar is: Web electron configuration of ruthenium (ru) [kr] 4d 7 5s 1. Electronic configuration of the krypton atom in ascending order of the.
Periodic Table Krypton Protons Periodic Table Timeline
Web what is the electron configuration of kr? 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. Web electron configuration of ruthenium (ru) [kr] 4d 7 5s 1. We can now add the remaining electrons for kr. 1s 2 2s 2 p 6 3s 2 p 6 d.
Element Krypton Electron Configuration What Is The Ground State
Web electron configuration of krypton is [ar] 3d10 4s2 4p6. The atomic mass of krypton is 83.798 u and its density is 3.75 g/l. We can now add the remaining electrons for kr. Its atomic number is 36 and it is located in the fourth row on the periodic. [ar]3d 10 4s 2 4p 6;.
What is the groundstate electron configuration of the element Kr
Web physical properties of krypton are mentioned below. 2, 8, 18, 15, 1. Chemspider is a free chemical structure database 1s2 2s2 2p6 3s2 3p6. This page shows the electron configurations of the neutral gaseous atoms in their ground states. In the atmosphere, 81 kr is chemically inert and has a long residence time; Web.
Iron(Fe) electron configuration and orbital diagram
Web 2.2 electron configuration [ar]4s 2 3d 10 4p 6. Kr has 18 electrons in the 4th energy level (n=4), so we can add them to the electron configuration of ar: [ar]3d 10 4s 2 4p 6; [ar] 3d 10 4s 2 4p 6. Once thought to be completely inert, krypton is known to form.
【5 Steps】Electron Configuration for Krypton in Just 5 Steps
Large number of spectral lines, strongest being green and yellow/ whitish emission ; Web electron configuration [kr] 4d 5 5s 1 cas number: Web electron configuration of ruthenium (ru) [kr] 4d 7 5s 1. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. Web krypton (kr), chemical element,.
What Is the Krypton(Kr) Electron Configuration ?
When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. Possible oxidation states are 0. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. There are two ways.
Kr Electron Configuration Long Form 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. Web the commonly used long form of the periodic table is designed to emphasize electron configurations. Know your orbitals know how many electrons each orbital can hold and their order. Web to help describe the appropriate notation for electron configuration, it is best to do so through example. For this example, we will use the iodine atom.
Los Alamos National Laboratory, U.s.
1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. [ar] 3d 10 4s 2 4p 6. About three times heavier than air , krypton is colourless, odourless, tasteless, and monatomic. Krypton has a total of 36 electrons second:
Web Next, We Need To Determine The Electron Configuration Of Kr's Noble Gas Predecessor, Which Is Ar (Atomic Number 18).
When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. Kr is the symbol for krypton, which is a noble gas. Web physical properties of krypton are mentioned below. The total number of electrons is the atomic number, z.
1S^ (2)2S^2 2P^6 3S^2 3P^6 4S^2 3D^10 4P^6 Or [Kr] Or [Ar] 4S^2 3D^10 4P^6 First:
Electronic configuration of the krypton atom in ascending order of the levels: Kr has 18 electrons in the 4th energy level (n=4), so we can add them to the electron configuration of ar: For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. We can now add the remaining electrons for kr.
Web Electron Configuration [Kr] 4D 5 5S 1 Cas Number:
Determine the number of electrons. This electron configuration shows that the last shell of krypton has eight electrons. Krypton is colorless, odourless and tasteless gas. For this example, we will use the iodine atom.