Which Pair Of Solutions Will Form An Effective Buffer - This problem has been solved!
Which Pair Of Solutions Will Form An Effective Buffer - Buffers usually consist of a weak. Web which pair of solutions will form an effective buffer? Web sap‑10.c.1 (ek) google classroom. 1.0 m hcn and 0.30 m kcn. 0.40 m hcl and 0.60 m naoh 0.10 m hf and 0.75 m mgf2 0.25 m h2so4 and 0.25 m na2so4 0.40 m hf and 0.30 m naf.
You'll get a detailed solution from a subject. If 0.025 moles of hcl is added to 325. Web ch3cooh and ca (ch₃coo)₂: Hcho 2 and nacho 2; The desired ph = 4.30, so: Web which pair should be used? The answer to this question is yes!
Which Pair Will Produce a Buffer Solution AllysonhasMccoy
When dissolved together, this pair will form a buffer. 0.35 m hf and 0.45 m hno2 0.50 m nh3 and 0.50 m ch3cooh 1.0 m hcn and 0.30 m kcn 2.0 m ch3cooh and 0.10 m ch3coona. Hcho 2 and nacho 2; A buffer solution is one in which the ph of the solution is.
Which Pair Will Produce a Buffer Solution AllysonhasMccoy
Web sap‑10.c.1 (ek) google classroom. The desired ph = 4.30, so: It has a basic ph and is used to maintain basic. What mass of nacn must be added to create. In the first approach, a certain amount of a weak acid (or weak base) is neutralized with. 1.0 m hcn and 0.30 m kcn..
Which of the following solutions, when c... Chemistry
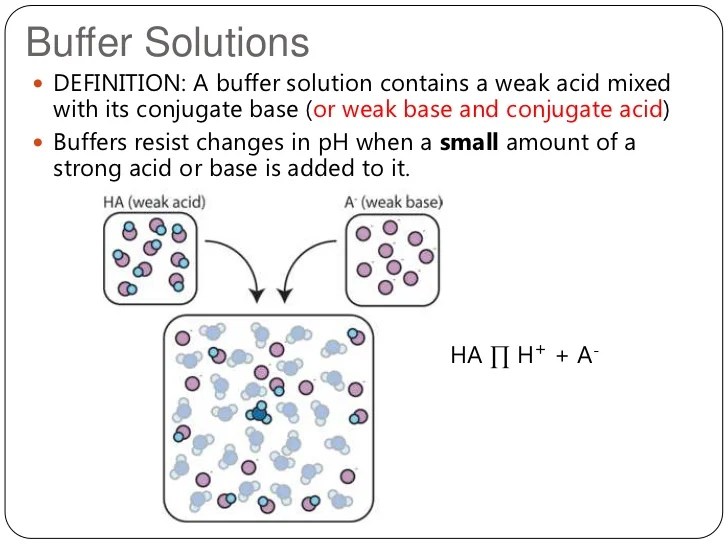
A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. “a buffer is an aqueous solution that resists changes in ph upon the addition of an acid or a base”. Web the most effective buffers contain equal concentrations of an.
Buffers Presentation Chemistry
Web sap‑10.c.1 (ek) google classroom. What is the ph of a buffer system that has 0.11 m ch3cooh and 0.15 m ch3coo−? The answer to this question is yes! “a buffer is an aqueous solution that resists changes in ph upon the addition of an acid or a base”. Web which pair of aqueous solutions.
Which Pair Will Produce a Buffer Solution AllysonhasMccoy
A buffer solution has 0.620 m hf and 0.710 m f−. In this video, we'll explore two common methods for preparing buffer solutions. Assume all are aqueous solutions. Buffers usually consist of a weak. What is the ph of a buffer system that has 0.11 m ch3cooh and 0.15 m ch3coo−? Web ch3cooh and ca.
Buffers, Buffer Components and Buffer Action Chemistry JoVE
Web which pair of aqueous solutions can create a buffer solution if present in the appropriate concentrations? Hcho 2 and nacho 2; 1.0 m hcn and 0.30 m kcn. It has a basic ph and is used to maintain basic. When dissolved together, this pair will form a buffer. Assume all are aqueous solutions. A.
2012 topic 18 2 buffer solutions
How do we define a buffer? A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. “a buffer is an aqueous solution that resists changes in ph upon the addition of an acid or a base”. It consists of a.
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint
Buffers usually consist of a weak. Web this mechanism involves a buffer, a solution that resists dramatic changes in ph. Web which pair of solutions will form an effective buffer? Web which pair of solutions will form an effective buffer? Web which pair of aqueous solutions can create a buffer solution if present in the.
Solved Which pair of compounds will form a buffer in aqueous
Buffers usually consist of a weak. Assume all are aqueous solutions. Web which pair of solutions will form an effective buffer? Web ch3cooh and ca (ch₃coo)₂: What amount of acid and base should you use to create the buffer? Chemistry of buffers and buffers in our blood. Web which pair of solutions will form an.
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint
A buffer that contains approximately equal amounts of a weak acid and. Web which pair of solutions will form an effective buffer? Web which pair of solutions will form an effective buffer? In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a.
Which Pair Of Solutions Will Form An Effective Buffer It consists of a solution of a. 0.75 m h3po4 and 0.45 m nah2po4. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. Web sap‑10.c.1 (ek) google classroom. 0.35 m hf and 0.45 m hno2 0.50 m nh3 and 0.50 m ch3cooh 1.0 m hcn and 0.30 m kcn 2.0 m ch3cooh and 0.10 m ch3coona.
Web Which Pair Of Solutions Will Form An Effective Buffer?
In this video, we'll explore two common methods for preparing buffer solutions. 0.35 m hf and 0.45 m hno2 0.50 m nh3 and 0.50 m ch3cooh 1.0 m hcn and 0.30 m kcn 2.0 m ch3cooh and 0.10 m ch3coona. Web which pair of solutions will form an effective buffer? A buffer has an effective ph range of one ph unit on either side of the pkₐ value for the weak acid.
A Buffer That Contains Approximately Equal Amounts Of A Weak Acid And.
1.0 m hcn and 0.30 m kcn. A buffer is effective when the ratio of the acid:base concentrations is. Web solution a is pure ‘water’, while solution b is a ‘buffer’. What amount of acid and base should you use to create the buffer?
Web A Buffer Solution Containing Relatively Large Quantities Of A Weak Base And Its Salt With A Strong Acid Is Called A Basic Buffer.
Web this mechanism involves a buffer, a solution that resists dramatic changes in ph. 0.40 m hcl and 0.60 m naoh 0.10 m hf and 0.75 m mgf2 0.25 m h2so4 and 0.25 m na2so4 0.40 m hf and 0.30 m naf. Web the most effective buffers contain equal concentrations of an acid and its conjugate base. Chemistry of buffers and buffers in our blood.
Ch 3 Nh 2 And Ch 3 Nh 3.
The answer to this question is yes! 0.75 m h3po4 and 0.45 m nah2po4. A buffer solution is one in which the ph of the solution is resistant to small additions of either a strong acid or strong base. What mass of nacn must be added to create.




.PNG)